About Syngenit Injectables Plus
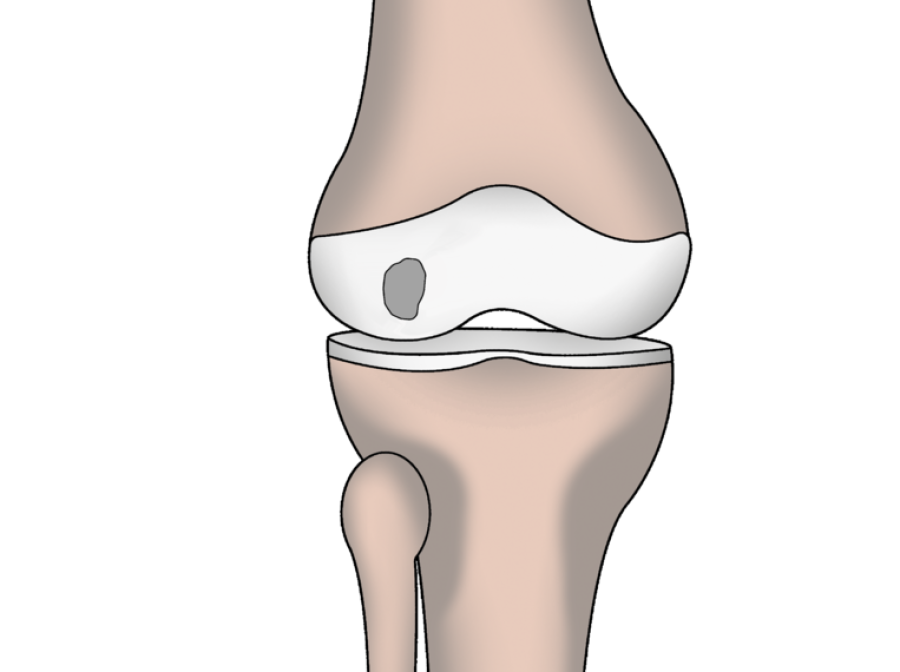
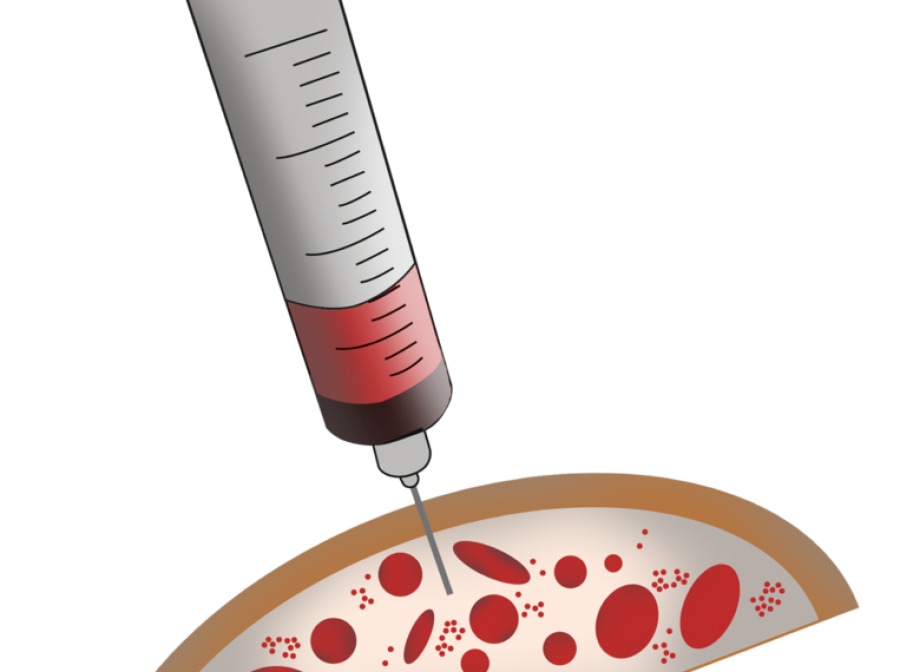
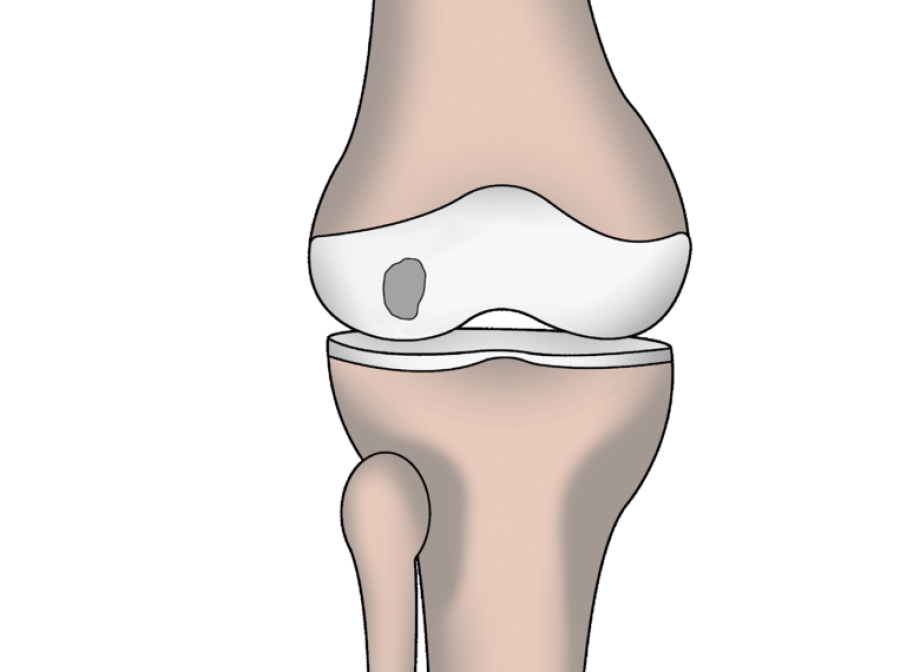
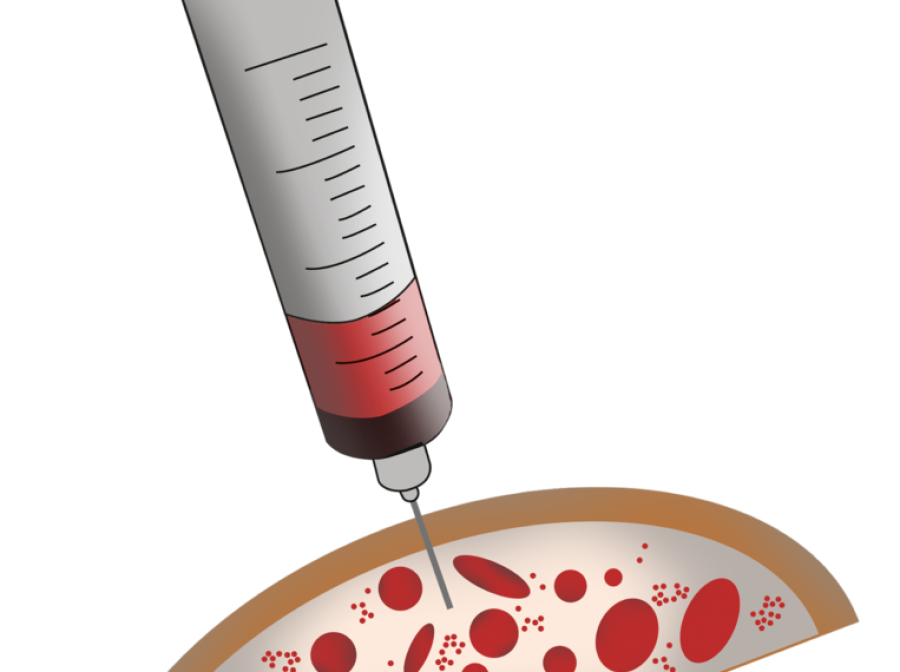
Syngenit™ Injectables Plus combines Bone Marrow Aspirate Concentrate (BMAC) with fibrin glue. It can be administered either as an injection or as a surgical procedure depending on the condition being treated.
Overview as a Percutaneous Procedure
Overview as a Surgical Procedure
Syngenit Injectables Plus Patient Leaflet
Syngenit Injectables Plus patient leafle[...]
Adobe Acrobat document [280.2 KB]
Adobe Acrobat document [280.2 KB]